neon neutrons|Isotopes of neon : Clark Element Neon (Ne), Group 18, Atomic Number 10, p-block, Mass 20.180. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content Eunys Mantes Abad scandal part 1. Eunys Mantes Abad scandal part 3 .
neon neutrons,Element Neon (Ne), Group 18, Atomic Number 10, p-block, Mass 20.180. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content
Learn about the number of protons, neutrons and electrons in neon, a colorless, odorless, inert monatomic gas with about two .

Neon has three stable isotopes: Ne (90.48%), Ne (0.27%) and Ne (9.25%). Ne and Ne are partly primordial and partly nucleogenic (i.e. made by nuclear reactions of other nuclides with neutrons or other particles in the environment) and their variations in natural abundance are well understood. In contrast, Ne (the chief primordial isotope made in stellar nucleosynthesis) is not known to b. Therefore, a neon atom has ten neutrons. The number of neutrons depends on the isotope of the element. The neon atom has three stable isotopes. Neon atom. This article discussed in detail how to .Neon is the 10th element in the periodic table and has a symbol of Ne and atomic number of 10. It has an atomic weight of 20.1797 and a mass number of 20. Neon has ten . The abundances of the naturally occurring isotopes of neon. Neon (10 Ne) possesses three stable isotopes: 20 Ne, 21 Ne, and 22 Ne. In addition, 17 radioactive .
Neon is the tenth element of the periodic table with the symbol Ne and the atomic number 10. It has three stable isotopes: 20 Ne, 21 Ne and 22 Ne. The most common isotope is neon-20 with 10 .
Interesting Facts about Neon. 0.0018 percent of Earth’s atmosphere is neon. Although it is relatively rare on our planet, neon is the fifth most abundant element in the universe. If you could gather all the neon from .neon neutrons Isotopes of neon Neon - Periodic Table. 10. Ne. Group. 18. Period. 2. Block. p. Protons. Electrons. Neutrons. 10. General Properties. Atomic Number. 10. Atomic Weight. 20.1797. Mass Number. 20. Category. Noble gases. Colorless. .The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of nucleons in an atom. The ratio of the average mass per atom . Neon-21 is a stable isotope containing 11 neutrons. 0.27% of natural neon is neon-21. 22 Ne Neon-22 is a stable isotope containing 12 neutrons. 9.25% of natural neon is neon-22. Neon filled neon sign .This is because the mass of the proton and neutron are each about 1 amu, while the mass of the electron is very small in comparison. mass number(A) = number of protons + number of neutrons (4.7.1) (4.7.1) mass number ( A) = number of protons + number of neutrons. Consider oxygen, which has an atomic number ( Z Z) of 8.Give the symbol of each isotope with the mass number as the superscript and the number of protons as the subscript, both written to the left of the symbol of the element. Solution: A The element with 82 protons (atomic .Prótons e nêutrons em Néon. Néon é um elemento químico com número atômico 10, o que significa que existem 10 prótons em seu núcleo. O número total de prótons no núcleo é chamado de número atômico do átomo e recebe o símbolo Z. A carga elétrica total do núcleo é, portanto, +Ze, onde e (carga elementar) é igual a 1,602 x 10-19 coulombs.
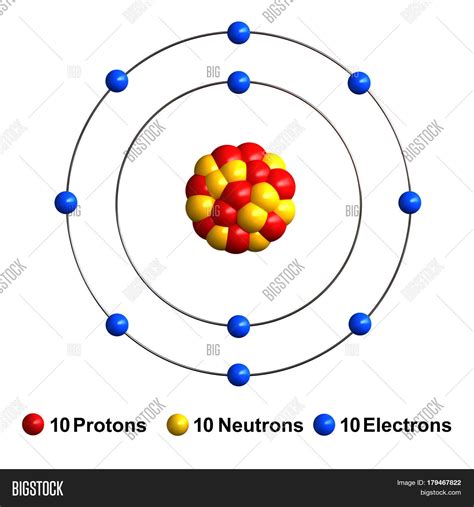
10 Facts About Element No. 10. Each neon atom has 10 protons. There are three stable isotopes of the element, with atoms having 10 neutrons (neon-20), 11 neutrons (neon-21), and 12 neutrons (neon-22). Because it has a stable octet for its outer electron shell, neon atoms have 10 electrons and no net electrical charge.
Just the facts. Atomic number (number of protons in the nucleus): 10. Atomic symbol (on the Periodic Table of Elements ): Ne. Atomic weight (average mass of the atom): 20.1797. Density: 0.0008999 .
Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Electrons are found outside the nucleus. Protons are positively charged and have a mass of about 1 u. .Neon has three naturally occurring isotopes. In a sample of neon, \(90.92\%\) of the atoms are \(\ce{Ne}\)-20, which is an isotope of neon with 10 neutrons and a mass of \(19.99 \: \text{amu}\). Another \(0.3\%\) of the atoms are \(\ce{Ne}\)-21, which is an isotope of neon with 11 neutrons and a mass of \(20.99 \: \text{amu}\).

Atomic Number – Protons, Electrons and Neutrons in Neon. Neon is a chemical element with atomic number 10 which means there are 10 protons in its nucleus.Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary .Follow-Up #1: Atomic properties of Neon. Q: how many protons, electrons, and neutrons are in neon- Joshua Doans (age 13) temple hills maryland. A: Hi Joshua, There are 10 protons, and 10 electrons in un-ionized neon. 90% of the neon atoms contain 10 neutrons. There are a few with 11 neutrons and around 9% with 12. 1 Answer. There are many isotopes of neon and each has a different number of neutrons. Neon always has 10 protons, but the number of neutrons varies with each isotope. The most common are 20 10N e (10 protons, 90.48% abundance), 21 10N e (11 protons, .27% abundance), and 22 10N e (12 protons, 9.25% abundance). Mass numbers of typical isotopes of Neon are 20; 21; 22. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known .
neon neutrons Fluorine has 9 protons, 10 neutrons and 9 electrons: 10: Neon has 10 protons, 10 neutrons and 10 electrons: 11: Sodium has 11 protons, 12 neutrons and 11 electrons: 12: Magnesium has 12 protons, .Isotopes of neon Fluorine has 9 protons, 10 neutrons and 9 electrons: 10: Neon has 10 protons, 10 neutrons and 10 electrons: 11: Sodium has 11 protons, 12 neutrons and 11 electrons: 12: Magnesium has 12 protons, .
Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure. Element Groups: Alkali Metals Alkaline Earth Metals . Basic Information Name: Neon Symbol: Ne Atomic Number: 10 Atomic Mass: 20.1797 amu Melting Point:-248.6 °C (24.549994 K, -415.48 °F) Boiling Point:-246.1 °C (27.049994 K, -410.98 °F) Number of .
Number of Neutrons: 10: Number of Electrons: 10: Melting Point-248.6° C: Boiling Point-246.1° C: Density: 0.901 grams per cubic centimeter: Normal Phase . Sir William Ramsay and Morris W. Travers: Common Compounds: There are no common compounds using neon. Interesting facts: It is the fourth most abundant element in the universe, but only .
Name of the isotope: Neon-20; Ne-20 Symbol: 20 Ne or 2010 Ne Mass number A: 20 (= number of nucleons) Atomic number Z: 10 (= number of protons) Neutrons N: 10 Isotopic mass: 19.99244018 (2) u ( atomic weight of Neon-20) Nuclide mass: 19.9869544 u (calculated nuclear mass without electrons) Mass excess: -7.04193 MeV Mass defect: .Neon is the 10th element in the periodic table and has a symbol of Ne and atomic number of 10. It has an atomic weight of 20.1797 and a mass number of 20. Neon has ten protons and ten neutrons in its nucleus, and ten electrons in two shells. It is located in group eighteen, period two and block p of the periodic table. Neon neutrons. Neutrons = atomic mass – atomic number. Neon neutrons. The atomic mass of neon is 20.1797, so we’ll take the roundup value as 20. And the atomic number of neon is 10. Subtract the atomic number (10) from the atomic mass (20). Hence, neon has a total of 20 – 10 = 10 neutrons.
neon neutrons|Isotopes of neon
PH0 · Neon – Protons – Neutrons – Electrons – Electron Configuration
PH1 · Neon – Protons – Neutrons – Electrons – Electron
PH2 · Neon Protons, Neutrons, Electrons – Complete Guide
PH3 · Neon Facts
PH4 · Neon Element Facts
PH5 · Neon (Ne)
PH6 · Neon (Ne)
PH7 · Neon
PH8 · Isotopes of neon